Quote:
Originally Posted by Digress
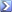
Where is an organic chemist when you need one ?
|
Since no organist chemist is around, I'll try my best with the chemistry lessons they give to pharmacy students in finland. Sorry if I sound confusing, I'm schooled in Finnish and english chemistry terms are somewhat new to me. Basing on the relative size and the angles of the molecylar bindings, I'd say that the atoms in the molecyle are Carbon (the big ones), Oxygen (There's one in a odd angle at the back in the right) and Hydrogen. It looks like a some sort of benzene derivative with some methyl groups added in and single ether group too.
I'd the guess the chemical in question is somewhat reactive and unstable due to the ether group. Probably not (too) toxic thought. It very likely is water solutible. I'm very unsure about this, but I think that it would be a liquid in room temperature.
Actually, looking at the picture in detail, I spot many inaccuracies. Many hydrogens are left out (why draw two hydrogens on the benzene-circle? Either you draw them all of them or leave them out). The top-most and most shiny carbon is missing two hydrogen! It might be a case of a double bonding with the carbons below and next to it, but there is nothing on the graphical representation of the bond in question to show this.
I'm sorry Swan, but apparently this random image on the net made to look like a molecyle is somewhat chemically inaccurate. One might even come to the conclusion that the artist hasn't studied chemistry (*GASP!*).
Yes, I'm shocked too.
I took the liberty of adding the missing hydrogen-atoms to the picture. I didn't do anything about the misrepresented molecylar bonds in the benzene circle, thought.
As for naming them, I'd say whatever goes. The real chemical name for the atoms would be pretty boring. Conquering "Carbon 1" wouldn't really bring out that epic feel that Dominions is famous for.
P.S(Yes! All those lessons were not in vain, for I was able to look smart on the internet!)
P.S.S (Why did I spend 45 minutes on this post? I could have been doing that breton update I've been meaning to do.)